

Complete parasite protection
Trifexis defends against heartworm disease, 3 intestinal worms and fleas.

Fast-Acting
Starts killing fleas within 30 minutes of administration, and kills new and existing adult worms all month long.

Easy to Give
Trifexis chewable tablets are beef-flavored for easy dosing.


How should I give Trifexis to my dog?
Give Trifexis with food for maximum effectiveness. Trifexis beef-flavored chewable tablets may be given from your hand, mixed in with food or placed in a treat. The tablet can be broken into smaller pieces as long as all pieces are administered in one sitting.

How often does my dog need to take Trifexis?
Continuous, year-round monthly treatment is recommended. Each box includes monthly reminder stickers for your calendar. Consult your veterinarian regarding your specific pet’s needs.

Can I combine Trifexis with other fleas & tick products?
Trifexis provides full flea protection for an entire month. Talk to your veterinarian before combining Trifexis with another flea infestation preventative or other medication.
*Trifexis, Elanco, and the diagonal bar logo are trademarks of Elanco or its affiliates. All other product names are brandmarks of their respective owners.
*Trifexis, Elanco, and the diagonal bar logo are trademarks of Elanco or its affiliates. All other product names are brandmarks of their respective owners.
Compare Our Coverage
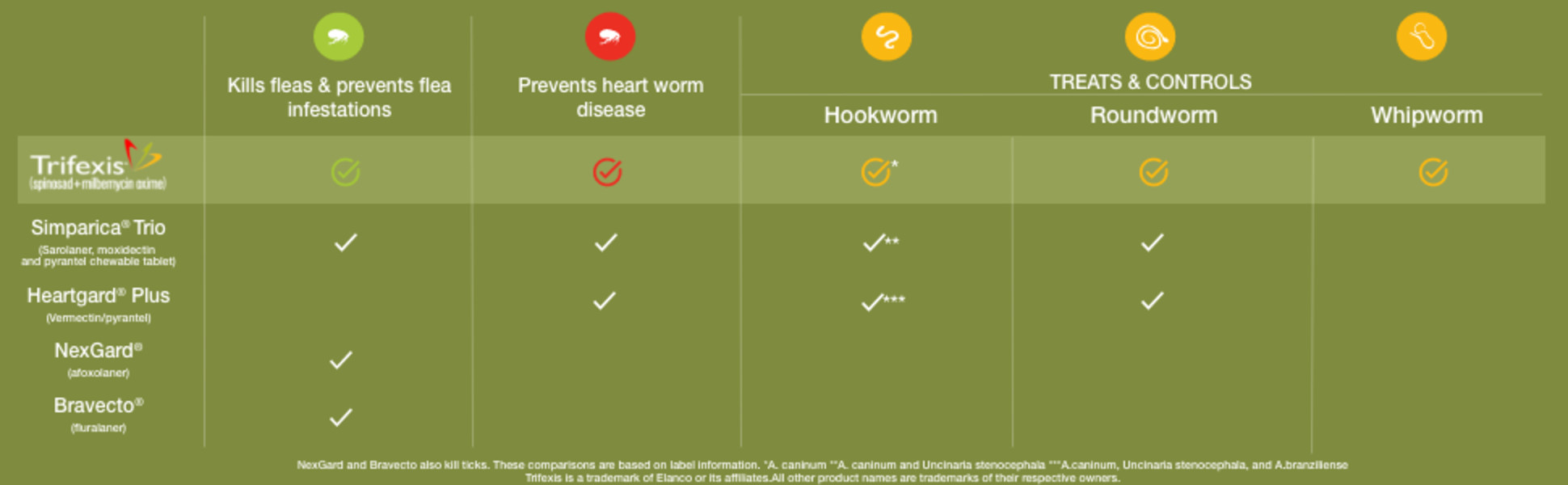
Indications
Trifexis prevents heartworm disease. Trifexis kills fleas and prevents flea infestations, and treats and controls adult hookworm, roundworm and whipworm infections in dogs and puppies 8 weeks and older and 5 pounds or more.
Important Safety Information
The use of ivermectin at higher than FDA-approved doses at the same time as Trifexis can result in serious side effects. Treatment with fewer than three monthly doses after the last exposure to mosquitoes may not provide complete heartworm prevention. Prior to administration of Trifexis, dogs should be tested for existing heartworm infection. Use with caution in breeding females. The safe use of Trifexis in breeding males has not been evaluated. Use with caution in dogs with pre-existing epilepsy. The most common adverse reactions reported are vomiting, decreased activity, itching, decreased appetite, and diarrhea. To ensure heartworm prevention, observe your dog for one hour after administration. If vomiting occurs within an hour of administration, redose with another full dose. Puppies less than 14 weeks of age may experience a higher rate of vomiting. For complete safety information, please see Trifexis product label or ask your veterinarian.